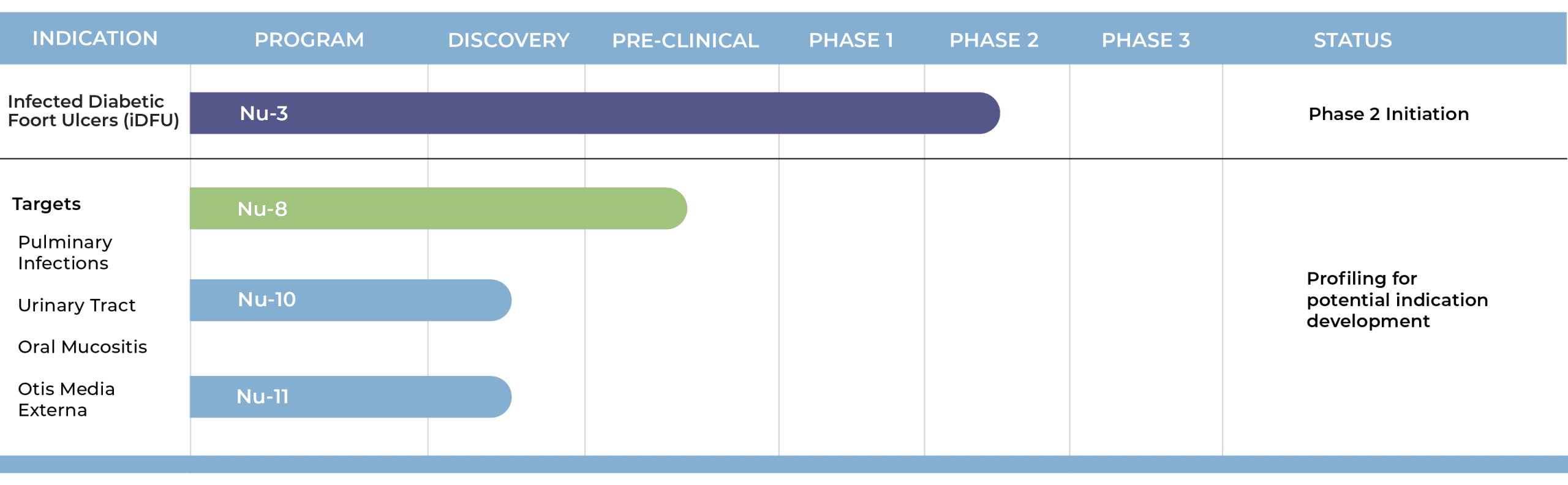
Pipeline
Lakewood-Amedex Biotherapeutics is focused on indications that can be targeted by locally acting antimicrobials such as skin and wound infections via topical application, complicated urinary bladder infections via direct instillation through a catheter for example in catheter associated infections, and lung infections via inhalation. Local administration has the advantage of rapid antimicrobial action, in addition to providing an attractive alternative to oral or intravenous drugs that require prolonged exposure to eradicate infections, and which can cause severe side effects as a result of their systemic exposure.
Lakewood-Amedex is advancing a gel formulation of the Bisphosphocin® Nu-3 into Phase 2 for the treatment of infected diabetic foot ulcers (iDFU) and is progressing several new compounds through the early-stage development to identify optimal treatment indications.
Lead Indication & Lead Candidate
Lead Indication – Infected Diabetic Foot Ulcers (iDFU)
830 million patients worldwide suffer from diabetes and close to 40 million are in the US. 1 Around one-third will develop a DFU in their life, of which about 50% will be infected at some point. Numerous patients experience more than one of these events in their life. Additionally, one-fifth of diabetic patients that have a foot ulcer will need an amputation, and infections, if not contained, are an important additional risk-factor alongside the presence of peripheral arterial disease, for future amputations. 2-3
Nu-3 Clinical Studies
An exploratory human trial on skin sensitization was conducted with Nu-3 solutions at 1 and 2% and no deleterious effects were observed. The same concentrations of Nu-3 solution were also studied in a small exploratory study in iDFU patients where positive trends in the reduction bacterial pathogens as well as a trend in wound area reduction were observed, supporting the further development of Nu-3. A formulation switch from solution to gel was identified as an improvement, and a gel formulation has been developed and patented.
A new Phase 2b clinical trial in iDFU has been designed and agreed upon with the FDA (NCT06020235 – ClinicalTrials.gov) comparing two different gel-concentrations (5% and 10%) each as either once-a-day or twice-a-day treatments in comparison to placebo. We anticipate this study will be preceded by a smaller phase 2a single blinded study comparing a 2%, 5% and 10% gel concentration to establish initial proof of concept in humans before the phase 2b study will be conducted.
New Discovery Program
Lakewood-Amedex Biotherapeutics is exploring new, potent molecules that have been recently discovered. The key focus of the discovery effort is to match the molecular properties of the newly discovered compounds to potential indications. The Bisphosphocin® class and the newly discovered compounds have molecular properties that allow for a broad range of different formulations to be developed including solutions, gels, nasal sprays, and nebulizers.
The indications being explored include complicated urinary tract infections via catheter delivery, bacterial lung infections, such as community and hospital acquired bacterial pneumonia (CABP & HABP) via nebulizer, cystic fibrosis via nebulizer, wound infections such as methicillin resistant Staphylococcus aureus (MRSA), and surgical infections.



