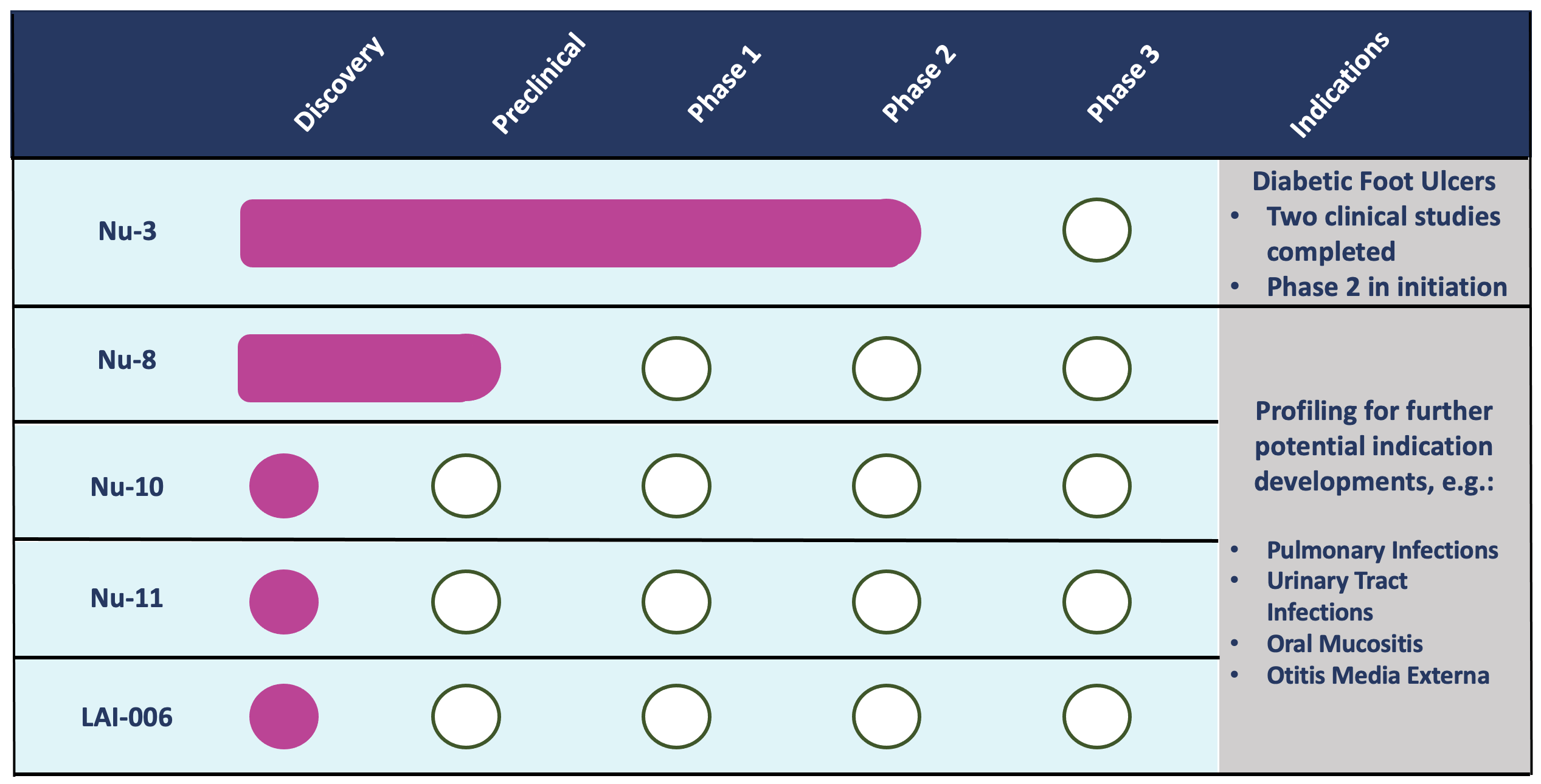
Pipeline
Lakewood-Amedex is focused on indications that can be targeted by locally acting antimicrobials such as skin and wound infections via topical application, bladder infections via direct instillation through a catheter, and lung infections via inhalation. Local administration has the advantage of rapid antimicrobial action, in addition to providing an attractive alternative to oral or intravenous drugs that require prolonged exposure to eradicate infections and which can cause severe side effects as a result of their systemic exposure.
Lakewood-Amedex is advancing a gel formulation of the Bisphosphocin Nu-3 into Phase 2 for the treatment of infected diabetic foot ulcers and is advancing several new compounds through the early stages of development to select the appropriate indication to be taken further into development (see Figure for portfolio status).